Yesterday, we started a lab with Cu/Fe. As a part of that lab, we’ll need to calculate how many moles of Cu/Fe were produced using the amount of grams of each. But what the heck is a Mole?
 Moles are a unit of measurement, sort of like “a dozen”. The term “dozen” refers to 12 of any item. A “mole” is sort of the same. Moles are equal to 6.02 x 1023 atoms. Thats 602,000,000,000,000,000,000,000… a lot of atoms. The real purpose of the ‘mole’ is to be able to do calculations of certain types of atoms, or between certain types of atoms. For example…
Moles are a unit of measurement, sort of like “a dozen”. The term “dozen” refers to 12 of any item. A “mole” is sort of the same. Moles are equal to 6.02 x 1023 atoms. Thats 602,000,000,000,000,000,000,000… a lot of atoms. The real purpose of the ‘mole’ is to be able to do calculations of certain types of atoms, or between certain types of atoms. For example…
To calculate grams from moles we use the FACTOR LABEL method. Let me show you what I mean…
Lets say you weighed your sample and have 105.5 g Cu atoms (Its important to label your values this way for future use; we’ll always add the element and “atoms” after the unit) and we want to know how many moles that is. well what do we know…
105.5 g Cu atoms -> ??? mol g Cu atoms
Well… lets think about this. Do we know how many grams are in 1 mol Cu atoms so we have something to compare this to??? Let’s look at our handy-dandy periodic table; find Cu; the atomic mass listed there is equal to the weight of 1 mol Cu atoms!
1 mol Cu atoms
63.5 g Cu atoms
So lets put this value into our formula and see if we can make this work…
105.5 g Cu atoms x 1 mol Cu atoms
1 63.5 g Cu atoms
We can cross out the “g Cu Atoms” units so the only unit remaining is “mol Cu atoms“. So…
105.5 x 1 mol Cu atoms = 1.66 mol Cu atoms
63.5
___________Easy! When you go from grams to moles you DIVIDE BY THE ATOMIC MASS!_______________
But, what if I want to go from moles to grams??? EASY!!! MULTIPLY BY THE ATOMIC MASS!
What is the weight of 2.5 moles of Carbon atoms?
2.5 mol C atoms x 12.0 g C atoms = 30.o g C atoms
1 1 mol C atoms
Since, 1 mole of any element = atomic weight of that element, you can flip the equation to work for you! Since we have moles on the top left of the formula, we want moles on the bottom right so they can cancel out!
Now you can do the Grams to Moles Conversions Worksheet (found on semester 1 Docs).




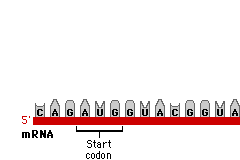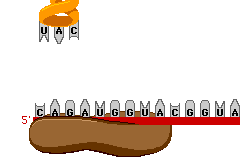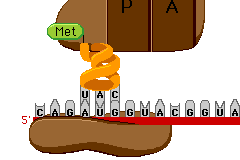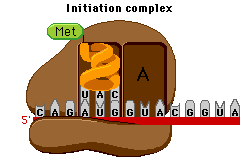
 Today in Biology we’ll start our last Unit of the semester… Protein Synthesis. Protein Synthesis is sometimes referred as the “Central Dogma” of Biology, meaning its the key idea.
Today in Biology we’ll start our last Unit of the semester… Protein Synthesis. Protein Synthesis is sometimes referred as the “Central Dogma” of Biology, meaning its the key idea.  Moles are a unit of measurement, sort of like “a dozen”. The term “dozen” refers to 12 of any item. A “mole” is sort of the same. Moles are equal to 6.02 x 1023 atoms. Thats 602,000,000,000,000,000,000,000… a lot of atoms. The real purpose of the ‘mole’ is to be able to do calculations of certain types of atoms, or between certain types of atoms. For example…
Moles are a unit of measurement, sort of like “a dozen”. The term “dozen” refers to 12 of any item. A “mole” is sort of the same. Moles are equal to 6.02 x 1023 atoms. Thats 602,000,000,000,000,000,000,000… a lot of atoms. The real purpose of the ‘mole’ is to be able to do calculations of certain types of atoms, or between certain types of atoms. For example…