Haha, I get it. Seriously though, we’re going to take some baby steps through the world of solutions. As a refresher, Solutions are a homogenous mixture (meaning it is evenly dispersed) of Solute and Solvent. The solvent is always the substance of larger amount when the two are mixed…
 For example, the Earth’s atmosphere is made up of several different types of gases. It is only about 20% oxygen but 76.5% Nitrogen! That being said, we would say that Nitrogen is the Solvent and all other gases are the solutes.
For example, the Earth’s atmosphere is made up of several different types of gases. It is only about 20% oxygen but 76.5% Nitrogen! That being said, we would say that Nitrogen is the Solvent and all other gases are the solutes.
There are different types of mixtures as well.
- Collids – are also Homogeneous, but the molecules of a collide do not settle or separate (like Milk)
- Suspensions – are heterogeneous meaning the parts of the mixture are not evenly dispersed (think Italian Dressing)
When it comes to solutions though, its a complete and even mixture. But not everything mixes so well. In chemistry, we say that “Like dissolves Like“. What that means is that things that have a smiler molecular make-up can mix together, like salt and water. we call these substances “miscible” because they are each polar molecules so they can mix. But Oil, which is non-polar and there for not like water, does not mix with water. We call substances that do not mix “immiscible“. But fear not oil fans, oil can mix with other non-polar substances because they are alike!
So what does mix with water? Well good solutions of water include Electrolytes. Electrolytes are compounds that can be broken down, or separated into ions. Salt, NaCl, is an electrolyte because it is split into Na+ and Cl– when placed in water. Non-electrolytes can also mix with water, but they do not separate. So solid (s) sugar molecules, since it cannot be split into ions, will stay as one molecule but will change form to become aqueous (aq). Why is that important? Well we calculate the number of particles created when a molecule is placed in solution with the V’Hoff factor (i). For example, Sodium Hydroxide, which is an electrolyte, dissociates (that means molecule made with ionic bonds is broken into ions) into Na+ and OH–. The V’Hoff factor would be i=2 because it splits into one of each of the ions. If it was MgCl2 , then i=3 because there would be one Mg+2 and two Cl–. For all Non-electrolytes, i=1 because the molecule does not separate. Like when sugar is placed in water… the sugar does not break apart, it stays together as C6H12O6. If it’s a covalenetly bonded molecule splitting into ions, it is called ionization.
What if we wanted to make Kool-aid and want to know exactly how much to place in 100g of water before it starts to just settle at the bottom. Then we need a Solubility curve…  Each line represents a different compound. Where the line does shows saturation, or the maximum amount of solute that can be dissolved in 100g of water before it starts to settles at the bottom. For Example, at 50°C we can dissolve 30g of K2Cr2O7 in 100g of H20. But at 90°C we can dissolve 69g of K2Cr2O7. We can also use this graph to determine if a solution is ‘supersaturated‘ (more than saturated – above the line) or ‘unsaturated‘ (less than saturated – below the line).
Each line represents a different compound. Where the line does shows saturation, or the maximum amount of solute that can be dissolved in 100g of water before it starts to settles at the bottom. For Example, at 50°C we can dissolve 30g of K2Cr2O7 in 100g of H20. But at 90°C we can dissolve 69g of K2Cr2O7. We can also use this graph to determine if a solution is ‘supersaturated‘ (more than saturated – above the line) or ‘unsaturated‘ (less than saturated – below the line).
For every solution there are two mole measurements we can determine. These measurements can help us determine how much solute or solvent is in a solution. These measurements are:
- Molarity (M) = molessolute/Literssolution
- Molality (m) = molessolute/kgsolvent
For these calculations, you can convert between moles and grams by either multiplying by molar mass (mol x MM = g) or by dividing by molar mass (g/MM = mol). If you need, click this link for a molar mass calculator. Luck There is so much more to study with solutions(colligative properties, ppm, & % solution), this is just the tip of the iceberg.
 I have had an absolutely blast working with each and every one of you. And it has been my absolute pleasure to get to know you. But, all good things must come to an end, and your end to this class is only a few clicks away.
I have had an absolutely blast working with each and every one of you. And it has been my absolute pleasure to get to know you. But, all good things must come to an end, and your end to this class is only a few clicks away.



 Each line represents a different compound. Where the line does shows saturation, or the maximum amount of solute that can be dissolved in 100g of water before it starts to settles at the bottom.
Each line represents a different compound. Where the line does shows saturation, or the maximum amount of solute that can be dissolved in 100g of water before it starts to settles at the bottom.  and complicated. Lucky for you, we’re going to simplify it to a point that is appropriate for a High School Biology student.
and complicated. Lucky for you, we’re going to simplify it to a point that is appropriate for a High School Biology student.
 Photosynthesis may seem like an easy topic, based on what you learned in middle school. But, as we go a little deeper into the processes of how Plants chemically create glucose from a gas, a liquid, and sunlight we find that it is a whole lot more interesting and complicated. Below is a graphic organizer to show the parts of the chloroplast, the reactants, the products, and the important molecules involved in both the Light & Dark Reactions.
Photosynthesis may seem like an easy topic, based on what you learned in middle school. But, as we go a little deeper into the processes of how Plants chemically create glucose from a gas, a liquid, and sunlight we find that it is a whole lot more interesting and complicated. Below is a graphic organizer to show the parts of the chloroplast, the reactants, the products, and the important molecules involved in both the Light & Dark Reactions.




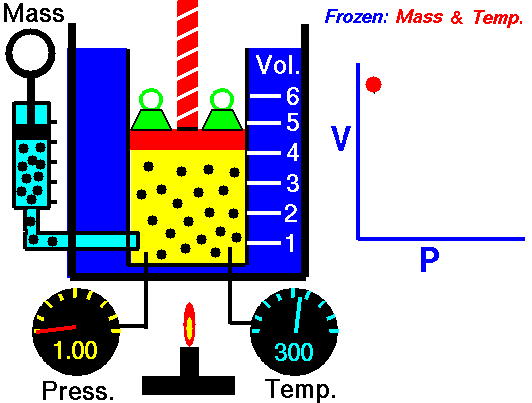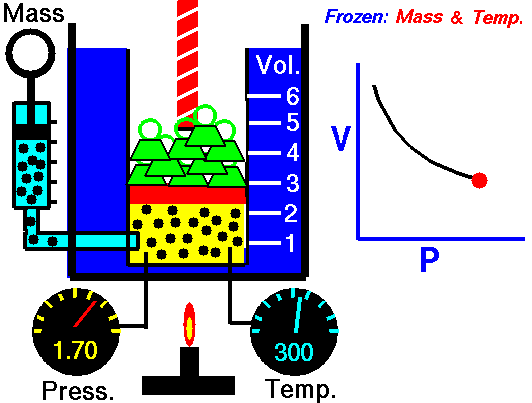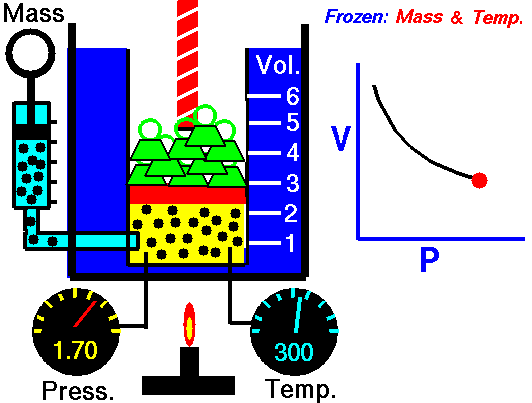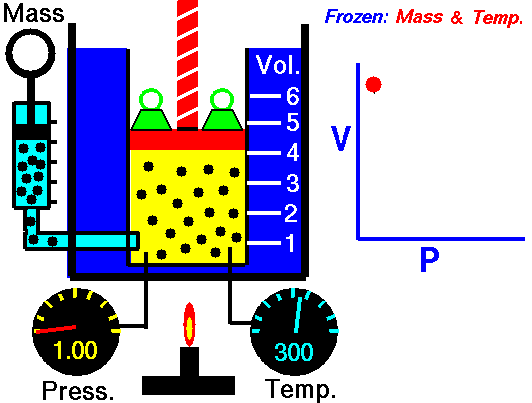
 Biology, here’s what we’ve got going today. We’re going to flip the classroom… sort of. In a true flipped classroom, I’d send you home with the assignment of taking notes so we could discuss the topics brought up in class the next day. BUT, we’re going to tweak that idea just a little bit. Here’s what I’d like you to do…
Biology, here’s what we’ve got going today. We’re going to flip the classroom… sort of. In a true flipped classroom, I’d send you home with the assignment of taking notes so we could discuss the topics brought up in class the next day. BUT, we’re going to tweak that idea just a little bit. Here’s what I’d like you to do…